Special section Extracranial germ cell tumors
Definition
Germ cell tumors (GCT) include a group of tumors that are highly heterogenous regarding their clinical behavior and broad spectrum of histologic appearance (Figure 1).
GCT arise from the primordial pluripotent germ cell and compose of tissue foreign to site of origin
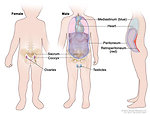
Localization:
-
gonadal
- ovary
- testis
-
extragonadal midline:
- brain, neck (Figure 2)
- mediastinum
- retroperitoneum
- sacro-coccygeal region
Both localization and histologic type of GCT are age-specific.
Clinical and biology behavior depends on:
- gender and age at diagnosis
- primary localizations
- histologic type
GCT may produce tumor markers and based on this are divided into secretoric or non-secretoric GCT.
Epidemiology
- GCT are rare tumors in children less than 15 years of the age, altogether they comprise approximately 3-5% of all cancers in this age group
- Annual incidence in adolescents is increasing and GCT account about 14% of all cancer; testicular tumors are the most common cancer in adolescent boys
-
Incidence GCT:
- 3.9 : 1 million (in children <15 let)
- 1.5/million gonadal GCT
- 2.4/million extragonadal GCT
-
An epidemiological analysis showed bimodal age distribution (Figure 3):
- The first peak is in infants aged <3 years: extragonadal (sacrococcygeal), testicular
- The second peak is in teenagers: gonadal, mediastinal
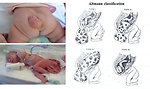
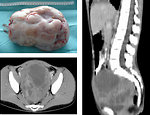
- Sacrococcygeal GCT constitute the most common neonatal tumors and teratoma is the predominate histologic type with slight female predominance (Figure 4)
- Distribution of gonadal and extragonadal tumor site is shown in Figure 5
- Some localisations of GCT (vaginal, sacrococcygeal) only develop during early childhood but not after the onset of puberty
Etiology and etiopathogenesis
Causes of germ cell tumors development are not clear. Some risk factors associated with germ cell tumors development have been recognized:
-
Genetic syndromes associated with sex chromosomal aberrations and abnormalities
- Klinefelter syndrome (47 XXY) – risk of mediastinal GCT
- Gonadal dysgenesis: Swyer syndrome ( 46,XY ) – risk of gonadal GCT; Turner syndrome (45, XO) – risk of gonadal GCT
-
Congenital anomalies of uropoetic tract
- cryptorchidism
- genito-urinary anomalies: hydrocele, inguinal hernia, hypospadia
There is no link to environmental factors and germ cell tumors development.
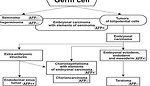
In 1975 Teilum postulated so-called holistic hypothesis of histogenesis of germ cell tumors. This hypothesis suggest that all different histologic types of germ cell tumors develop from primordial pluripotent germ cell that is capable of differentiation along the germ line and into embryonic and extraembryonic tissues (Figure 6).
Clinical presentation and symptoms
Depend on:
- localization of the tumor
- age
- histologic type of the tumor and its biology behavior (Figure 7)
The first symptom is usually palpable or visible tumor mass.
Local symptoms are based on localization of the tumor
-
sacrococcygeal tumors:
- visible mass of sacrococcygeal area (Figure 8)
- gluteal furrow deformation
- constipation, bowel obstruction
- urinary bladder obstruction
- neurologic abnormalities of lower extremities
-
retroperitoneal tumors:
- asymptomatic for period of time
- neurologic abnormalities due to spinal compression
-
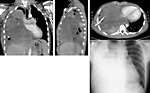
mediastinal tumors:
- cough, dyspnea, wheezing (Figure 9)
- chest wall pain, tiredness
-
ovarian tumors:
- asymptomatic for long period of time
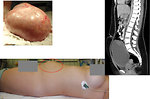
- palpable/visible abdominal tumor mass (Figure 10 and 11)
- abdominal pain, nausea
- menstruation problems
-
testicular tumors:
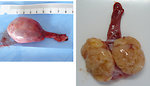
- painless swelling of the testis (Figure 12 and 13)
- palpable lump in the testis
Systemic symptoms (fever, weakness, weight loss, sweating, itching etc.) are rare.
Gonadal tumors can produce hormones and symptoms are caused by paraneoplastic hormonal production leads to:
- virilism in girls
- pseudopubertas praecox
- gynecomastia in boys
Diagnostic procedures
History: we have to ask for duration of any symptoms – pain, abdominal distension, passing stool and urine, cough, breathing difficulties etc. Menarche is very important information in pubertal girls as well as irregular menstruation cycle
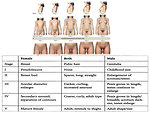
Physical examination: detailed physical examination, assessment of pubertal development according to Tanner scale (Figure 14). To assess symptoms of precoccious puberty, virilism, gynecomastia in boys.
Imaging studies:
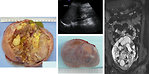
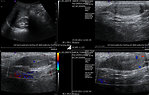
- Ultrasound is most often used for an initial evaluation of patients with abdominal or pelvic mass and will differentiate cystic form from solid masses. Is the first study for diagnosis of testicular tumors to see the size, character and vascularisation of the testicular lump (Figure 15).
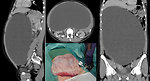
- CT/MRI: is used for more detailed informations of mediastinal, abdominal and pelvic masses - origin of the tumor, its size, metastatic involvement of lymph nodes, adjacent organs infiltration. It is mandatory for further management, treatment plan and timing of definitive surgery (Figure 16 a 17).
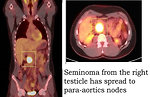
- 18 FDG PET scan: may be helpful to identify dissemination of germ cell tumor. In a case of residual mass FDG PET scan has the potential to detect active vital malignant disease and distinguish it from fibrotic tissue. FDG PET has demonstrated efficacy for monitoring therapeutic response. In the follow-up of GCT patients, the ability of FDG-PET to identify suspected recurrences is high (Figure 18).
Tumor markers: certain histologic variants of GCT secrete the tumor markers – AFP (α-fetoprotein) and β-HCG ( β-human chorionic gonadotropin). The production of these markers has recognized diagnostic value.
- AFP is a major serum protein of the fetus, it is produced in the embryonic liver and yolk sac and in the gastrointestinal tract. After delivery AFP serum levels gradually falling to reach a normal level at approximately age 1 year. The highest AFP levels are seen in endodermal sinus (yolk sac) tumors, immature teratoma, mixed GCT and in part of embryonal carcinoma. (Figure 19)
- Β-HCG is a glycoprotein normally synthesized during pregnancy by syncytiotrophoblast of the placenta. Elevation of β-HCG in patients with GCT implies the presence of syncytiotrophoblast, such as choriocarcinoma, dysgerminoma and occasionally embryonal carcinoma. Pure teratomas are not associated with AFP or β-HCG production.
-
Other markers:
- LDH is not specific for GCT, but may correlate with disease activity and tumor burden
- CEA (carcinoembryonic antigen) elevated serum levels are reported in ovarian tumors, also can be detected in mature teratomas
- Ca-125 is related to ovarian tumors and is the marker of peritoneal irritation or involvement
Hormonal profile: is important to evaluate in pubertal girls and boys to measure hormonal production and activation of hypophyseal – gonadal axis during puberty and in all cases with clinical symptoms of virilism, delayed puberty or pseudopubertas praecox.
Genetic tests: in a cases of ovarian tumors suspected of intersex disorder are important genetic tests (karyotype and SRY) as well as in adolescent boys with mediastinal tumors ( to exclude Klinefelter syndrome).
Differential diagnosis
Differential diagnosis depends on:
- localization of the tumor
- age and gender of the child
Sacrococcygeal tumors: are typical for neonates and infants. In differential diagnosis we need to exclude:
- neuroblastoma
- soft tissue sarcoma (rhabdomyosarcoma)
- congenital hamartoma
Ovarian tumors: typical for pubertal and adolescent age
- congenital anomalies (polycystic ovaries, endometriosis)
- inflammatory changes
- ovarian torsion
- other malignant tumors (sarcomas, epithelial tumors – carcinomas and granulosa cell tumors)
- pregnancy
Testicular tumors: bimodal age curve (small boys up to 5 years with teratoma or pure yolk sac tumor and adolescent boys with mixed germ cell tumors):
- varicocele, hydrocele
- orchitis
- testicular torsion
- paratesticular tumors (rhabdomyosarcoma)
- secondary infiltration of the testis by hematological malignancies (leukemia/lymphoma)
- Leydig cell tumors
Retroperitoneal tumors: are typical for pre-school children
- benign tumors
- congenital anomalies ( advanced hydronephrosis, polycystic kidneys)
- other malignant tumors ( neuroblastoma, rhabdomyosarcoma, rarely paraspinal Ewing sarcoma or lymphoma)
Mediastinal tumors: are more frequent in adolescent age, predominantly boys:
- other malignant tumors (Hodgkin and non-Hodgkin lymphoma, leukemia, rhabdomyosarcoma, Ewing sarcoma, thymoma, carcinoma)
- benign tumors (neurofibroma, ganglioneuroma)
- reactive mediastinal lymphadenopathy or autoimmune disease
Therapy
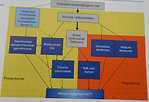
Treatment strategy depends on:
- primary localization of the tumor (site of origin)
- extent of disease (stage)
- age of the patient
- tumor markers elevation
These parameters are crucial for risk stratification and management (Figure 20)
Surgery:
- Surgery is a mainstay in the treatment of GCT
- For mature teratomas in any site complete surgical resection is curative approach
- For other histologic types of GCT radical resection of the tumor mass is indicated, if possible
- In gonadal localization of GCT (ovaries, testicles) conservative surgery (unilateral salpingo-oophorectomy or unilateral orchiectomy with high ligation of spermatic cord) is standard of care in order to preserve fertility
- Delayed surgery (after neoadjuvant chemotherapy) is indicated in case of initially inoperable bulky disease in order to avoid mutilation surgery
- For sacrococcygeal localization complete surgical resection along with removal of the entire coccyx is mandatory (Figure 21)
- In testicular tumors with retroperitoneal lymph nodes involvement dissection of retroperitoneal lymph nodes is not indicated
- If residual tumor mass persist after chemotherapy, it is mandatory to resect it irrespective of tumor markers serum levels
Chemotherapy:
- Chemotherapy is of the key importance , because malignant GCT may respond dramatically to neoadjuvant chemotherapy
- Prognosis of GCT improved significantly with development of cisplatin based chemotherapy
- Neoadjuvant chemotherapy may allow a patient to be spared mutilating surgery
- Duration and intensity of chemotherapy is based on risk classification (location of the tumor, extent of disease -clinical stage and age of the patient)
- High dose chemotherapy with autologous peripheral hematopoietic stem cell transplantation is currently not a standard treatment for recurrent pediatric GCT, only as a part of clinical trials
Radiotherapy:
- Radiation sensitivity of GCT correlates with histologic type
- Historically radiotherapy has been used for dysgerminomas, seminomas, but currently the role of radiotherapy has been reduced by the advent of cisplatin based chemotherapy in order to prevent late toxicity
- Radiotherapy may be used in palliative intention for pain relief in metastatic GCT
Prognosis and outcome
Germ cell tumors are curable types of cancer and prognosis improved dramatically with definition of surgical guidelines and cisplatin-based chemotherapy.
5 years overall survival:
-
localized disease > 95%
- stage I 97%
- stage II 95.7%
-
advanced disease > 80%
- stage III 90%
- stage IV 85%
Also recurrent GCT (10–15%) are still curable in high percentage (75–80%).
Author: Viera Bajčiová, MD, PhD