Special section Acute myelocytic leukemia
Definition
- Acute myeloid (myelogenous, myelocytic, myeloblastic) leukemia (AML) consists of a group of malignant disorders characterized by the replacement of normal bone marrow with abnormal, primitive hematopoietic cells.
- Acute leukemia is believed to begin in a single somatic hematopoietic progenitor that transforms to a cell incapable of normal differentiation. Many of the leukemic cells no longer possess the normal property of apoptosis, or programmed cell death. As a result, they have a prolonged life span and are capable of unrestricted clonal proliferation. Because transformed cells lack normal regulatory and growth constraints, they have favorable competitive advantage over normal hematopoietic cells. The result is the accumulation of abnormal cells with qualitative defects. The major cause of morbidity and mortality is the deficiency of normally functioning, mature hematopoietic cells rather than the number of malignant cells.
Epidemiology
- Acute myeloid leukemia accounts for nearly 1/3 of newly diagnosed cases of leukemia in children.
- Although 1 in every 3 newly diagnosed leukemias is acute myeloid leukemia, the ratio of acute myeloid leukemia to ALL rapidly decreases until adolescence. During adolescence, the rate increases to account for nearly 50% of all new diagnoses of leukemia.
- Incidence of approximately seven occurrences per 1 million children annually.
- The long-term survival rate for pediatric patients with acute myeloid leukemia is nearly 70%.
- Acute myeloid leukemia accounts for about 35% of childhood deaths from leukemia. Mortality is a consequence of resistant progressive disease or treatment-related toxicity.
- Male and female distributions are nearly equal at all ages.
Etiology/pathophysiology
Cause of acute myeloid leukemia is unknown in most patients
Risk factors for developing AML are:
Radiation exposure
Exposure to toxins
- Exposure to toxic chemicals that cause damage to bone marrow, such as benzene and toluene (used in the leather, shoe, and dry cleaning industries), is associated with leukemia in adults. Direct evidence of this effect in children has not been established. Exposure to pesticides has been noted to increase the risk of acute myeloid leukemia.
Exposure to drugs
- A compelling association has been observed after treatment with antineoplastic cytotoxic agents, particularly alkylating agents such as procarbazinethe nitrosoureas, cyclophosphamide, melphalan, and the epipodophyllotoxins etoposide and teniposide. Patients receiving these agents to treat malignancies (eg, Hodgkin disease) have a significantly increased risk of developing a preleukemic syndrome that ultimately transforms into overt acute myeloid leukemia, especially if the agents are administered with radiation therapy.
Genetic factors and syndromes
- Children with Down syndrome (trisomy 21) have a 15-fold increased risk of developing leukemia, most commonly acute megakaryoblastic leukemia, compared with the general population. The risk of megakaryoblastic leukemia in Down syndrome is approximately 400 times greater than it is in the rest of the population. Children with Down syndrome who have transient myeloproliferative syndrome as neonates, a condition often indistinguishable from acute leukemia, also have a high risk of developing acute leukemia in subsequent years.
- Patients with inherited disorders, such as Shwachman-Diamond syndrome, Bloom syndrome, Diamond-Blackfan anemia, Fanconi anemia, dyskeratosis congenita, and Kostmann syndrome, also have an elevated risk of developing leukemia.
- Children with neurofibromatosis type I also appear to be at increased risk for developing acute myeloid leukemia.
Classification of AML
The French-American-British classification system (FAB) recognizes 7 primary types of AML (M1-M7), which can usually be established by morphology and additional marrow studies (Figure 1).
Table 1. FAB classification of AML
|
FAB subtype |
Name |
AML patients (%) |
|
M0 |
Undifferentiated acute myeloblastic leukemia |
5% |
|
M1 |
Acute myeloblastic leukemia with minimal maturation |
15% |
|
M2 |
Acute myeloblastic leukemia with maturation |
25% |
|
M3 |
Acute promyelocytic leukemia (APL) |
10% |
|
M4 |
Acute myelomonocytic leukemia |
20% |
|
M4eos |
Acute myelomonocytic leukemia with eosinophilia |
5% |
|
M5 |
Acute monocytic leukemia |
10% |
|
M6 |
Acute erythroid leukemia |
5% |
|
M7 |
Acute megakaryocytic leukemia |
5% |
Recent improvements in identifying the molecular genetics and pathogenesis of AML have been implemented in the new World Health Organization (WHO) classification of AML:
- AML with characteristic cytogenetic translocations
- AML with multilineage dysplasia
- AML and myelodysplasia syndromes secondary to therapy
- AML not otherwise categorized
Signs and symptoms
Signs and symptoms of pediatric acute myelocytic leukemia (AML) can be divided into the following:
Symptoms due to a deficiency of normally functioning cells:
- Cytopenias: Can result from a deficiency of normally functioning cells
- Anemia: Characterized by pallor, fatigue, tachycardia, and headache
- Hemorrhage: Most commonly, easy bruising, petechiae, epistaxis, gingival bleeding
- Fever: Should initially always be attributed to infection
Symptoms due to the proliferation and infiltration of the abnormal leukemic cell mass and infiltrative disease include the following:
- Extramedullary infiltration: Most commonly in the reticuloendothelial system
- Mediastinal mass: May cause symptoms of respiratory insufficiency or superior vena cava syndrome
- Abdominal masses: May cause pain or obstruct the GI or urogenital tracts
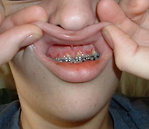
- Gingival hyperplasia, CNS infiltration: Often associated with monoblastic leukemia (Figure 2)
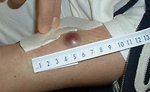
- Skin infiltration (Figure 3)
Constitutional symptoms
Children may not have well-known symptoms of leukemia, such as adenopathy, overt bleeding, and serious infections. Nonspecific symptoms, such as fatigue, irritability, fevers, and bruising, are common in childhood and might not be recognized as symptoms of leukemia, thus delaying a diagnosis of leukemia. Persistence of these symptoms should prompt further investigation.
Diagnostic tests
The hallmark of AML is the reduction or absence of normal hematopoietic elements. Anemia is usually normocytic, with a lower-than-expected reticulocyte count for the hemoglobin level. The decrease in hemoglobin levels can range from minimal to profound.
Laboratory tests used in patients with AML include the following:
- Blood counts with differential: WBC counts may be decreased or elevated; platelet counts usually low
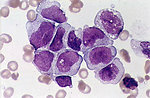
- Blood smears: Primitive granulocyte/monocyte precursors observed; Auer rods present in specimens of circulating blood from many AML patients but particularly prominent in pediatric APL
- Blood chemistries: Frequently elevated serum uric acid, serum muramidase (lysozyme), LDH levels
- Blood and urine cultures: Always obtain in a child with fever and leukemia
- Coagulation tests: Perform with initial diagnosis for evidence of DIC indicating APL
- Histochemical staining: Standard Wright-Giemsa stains and histochemical stains to differentiate the various acute leukemias
- Immunophenotyping: To further characterize leukemic cells for different cell lineages and stages of development
- Cytogenetic testing: To confirm the diagnosis and for prognostic purposes
- HLA typing: To identify HLA–matched family donors for possible BMT or HSCT in high-risk patients
- Bone marrow examination: To establish the diagnosis of AML
- Lumbar puncture and CSF examination: For diagnostic and therapeutic purposes
Differentials diagnosis
- Aplastic anemia
- Drug-induced pancytopenia
- Viral-induced pancytopenia
- Systemic lupus erythematosus
- Disseminated neuroblastoma or alveolar rhabdomysarcoma
- Transient myeloproliferative syndrome in Down syndrome
Prognostic factors
Various molecular abnormalities have an impact on outcome:
Cytogenetic abnormalities
Leukemia cells demonstrate clonal cytogenetic abnormalities in more than 85% of patients. These changes are often unique to the subtype. For example:
- t(15;17) translocation is nearly always found in patients with APL
- t(8;21) is most commonly found in those with myeloblastic leukemia.
- some of the cytogenetic abnormalities have now been shown to confer either greater risk of recurrent disease (eg, monosomy 7 and monosomy 5) or lower risk (eg, t[8;21] and inv[16]/t[16;16]).
Molecular studies
In addition to the established prognostic cytogenetic abnormalities, increasing evidence has revealed various molecular abnormalities that have an impact on outcome.
- The presence of the FLT3/ITD mutation, a receptor tyrosine kinase mutation, has been established as a predictor of worse outcome.
- Another gene affecting prognosis is the nucleophosmin (NPM1) mutation. The presence of this mutation has been shown to confer a favorable prognosis for event-free survival.
- The presence of MLL gene is usually an unfavorable prognostic marker.
- The presence of the Wilms tumor gene (WT1) is also an adverse prognostic marker, with patients often failing to achieve complete remission.
Therapy
The treatment of AML is directed toward 2 goals:
- destroying the leukemic cells as rapidly as possible and preventing the emergence of a resistant clone
- supporting the patient through long periods of pancytopenia until their bone marrow achieves hematologic remission and is again producing normal hematopoietic cells.
Chemotherapy
Chemotherapy used in managing AML includes the following medications (Figure 4):
- Chemotherapeutic drugs: Cytarabine (cytosine arabinoside), fludarabine, daunorubicin (daunomycin), etoposide, amsacrine, 6-thioguanine, cyclophosphamide, mitoxantrone, tretinoin, arsenic trioxide, L-asparaginase, gemtuzumab ozogamicin
Nonpharmacologic therapy
AML may also be managed with nonpharmacologic treatments such as the following:
- Allogeneic or autologous BMT following chemotherapy and irradiation: May reduce relapse rates but doesn’t always improve overall survival
- Radiation treatment: Primarily to treat chloromas and other masses pressing on a vital structure and that may imminently cause irreversible damage; craniospinal irradiation for persistent CNS leukemia
Treatment of acute promyelocytic leukemia
The discovery of effective maturation agents has altered the approach to treating APL. All-trans retinoic acid (ATRA) can effectively induce remission in most newly diagnosed APLs with the myelosuppressive effects of chemotherapy.
Another approach that is being investigated in clinical trials is the use of arsenic trioxide, which is highly active in newly diagnosed and relapsing APL. It effectively induces remissions in 85% of patients who have a relapse.
Novel therapies
The frequent occurrence of mutations in receptor tyrosine kinase genes (FLT3 and KIT) has generated interest in the development of tyrosine kinase inhibitors.
The presence of frequent mutations in genes involved in DNA methylation and chromatin modification, as well as the identification of new epigenetic targets by global proteomic approaches and functional screens, have informed another exciting and rapidly expanding therapeutic area — the development of new epigenetic therapies.
Antibody therapy for AMLmonoclonal antibodies targeting CD33, either with the use of antibody–drug conjugates or bispecific antibodies (anti-CD33 and CD3). Ttargeting antigens such as CD123, the transmembrane alpha chain of the interleukin-3 receptor, that are preferentially expressed on leukemic stem cell.