Late effects of treatment of childhood cancer
Survivors of childhood cancer
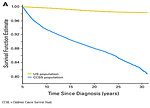
During the past decades dramatic progress and survival improvement has been made in pediatric oncology. Number of “cured” patients and childhood cancer survivors in the population continues to grow. It is indicated that one of the 600 young adults (up to 39 years of the age) is a childhood cancer survivor and 25% of childhood cancer survivors is younger than 35 years (Figure 1).
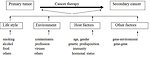
The most of childhood cancer survivors experience late effects secondary to their cancer or its treatment. Estimates are that as many as two-thirds of survivors will experience at least one late effect, with perhaps 25% of survivors experiencing more that one late effect of cancer treatment. One of 4 survivors experience severe or life-threatening late effects (Figure 2).
Chemotherapy, radiation therapy, and surgery may all cause late effects involving any organ or system of the body. Exposure to therapeutic agents during the rapid and dramatic physiologic and psychologic changes occurring from infancy to early adulthood can result in specific organ damage and alteration of normal patterns of growth and development.
Late effects of surgery with implications for survivorship may include amputation (bone or locally advanced soft tissue sarcomas), eye enucleation (due to retinoblastoma) or growth abnormalities.
Late effects of chemotherapy and radiation are common due to aggresiveness of pediatric treatment protocols and may be mild or severe. As many as two thirds of survivors will experience a late effect of chemotherapy or radiotherapy.
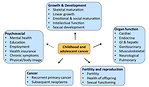
The most common late effects of childhood cancer include those that are neurocognitive and psychological, cardiopulmonary, endocrine, hearing loss, musculoskeletal, fertility impairement and the most serious late effect is second malignant cancer (Figure 3).
The emergence of late effects depends on many factors:
- Age of the patient at the time of therapy (younger age has the greater risk of severe and progressing late effects)
- Type, localisation and extent of primary cancer
- Dose and volume of radiotherapy
- Type and cumulative dose of chemotherapy
- Combination of chemo and radiotherapy
- Comorbidities (other chronic illnesses, inborn errors etc)
- Genetic predisposition
Cardiotoxicity
Anthracyclines have been used in the treatment of several childhood cancers (Hodgkin’s and non-Hodgkin’s lymphomas, soft tissue sarcomas, ALL, Wilms’ tumor, neuroblastoma, hepatoblastoma) for many years. Their role in the risk of developing late cardiotoxicity is known and dose-dependent:
- Cumulative dose > 600 mg/m2 – 36% risk of cardiac event
- Cumulative dose 551– 600mg/m2 – 18% risk
- Cumulative dose 500– 550mg/m2 – 4% risk
Additional independent risk factors for cardiotoxicity are:
- gender (females have higher risk)
- young age at anthracyclines treatment
- concurrent/concomitant treatment with chest radiotherapy (Figure 4)
- cardiac inborn errors
Clinical research regarding cardioprotective drugs (e.g., dextrazoxane) is under investigation.
Most survivors who develop left ventricular dysfunction (detectable on echocardiography) after treatment with an anthracyclines will likely remain asymptomatic. Within the first 10 years after treatment, about 5 to 20% of survivors will have overt congestive heart failure. The number of patients with severe left ventricular dysfunction increases with the duration of follow-up.
Late cardiac failure may be induced in pediatric cancer survivors with anthracyclines chemotherapy by alcohol consumption, physical inactivity or rapid growth. In female survivors, the initial presentation of congestive heart failure may be clinically presented during pregnancy.
Periodic monitoring of left ventricular function in two years intervals (echocardiography and stress echocardiography) of asymptomatic survivors who were treated with moderate to high doses of athracyclines is recommended.
Pulmonary toxicity
Late pulmonary toxicity of cancer therapy mainly affects pulmonary interstitium. All three treatment modalities can lead to pneumonitis or interstitial pulmonary fibrosis.
Surgery: may affect lungs by formation of postoperative fibrotic changes, adhesions and reduction of functional lung parenchyma (mediastinal germ cell tumors, neuroblastoma or chest wall sarcomas).
Chemotherapy: chemotherapy agents (bleomycin, carmustine, high dose cyclophosphamide and methotrexate, busulphan, melphalan) may cause lung fibrosis with reduction of lung function.
Radiation therapy: chest and thoracic irradiation can adversely affect lung function several ways:
- by inhibiting chest wall growth that may in turn diminish lung volume
- by formation of lung fibrosis
- by formation of pneumonitis
Risk of severe pulmonary toxicity is higher in patiens with some comorbidities (astma, cystic fibrosis or other preexisting pulmonary disease) and in patients which lungs are primary affected by the tumor (Langerhans cell histiocytosis, massive pulmonary metastases).
Periodic assessment of pulmonary function (spirometry) in two years intervals is generally recommended for all pediatric cancer survivors with risk factors mentioned above. Very important is lifestyle modification (an absolut ban of smoking, to reduce exposition to passive smoking, to ovoid deep diving).
Ototoxicity
Despite hearing loss is not a life threatening late effect of cancer treatment, it can significantly delay speech and language development, specially at young age (less than 3 years). Ototoxicity and hearing loss may have negative impact on education and psychosocial development and quality of life of pediatric cancer survivors.
Early identification of hearing loss in young children is critical for optimizing normal speech and language development. Therefore initial examination (audiometry) is essential as part of initial work up before starting chemotherapy with ototoxic drugs. Monitoring of ototoxicity is an important component of management of pediatric cancer patients who are at risk to develop ototoxicity.
Risk factors associated with risk of ototoxicity include:
- Platinum-based chemotherapy
- Concomitant cranial radiation therapy
- Other ototoxic medication (diuretics, vancomycin, antimycotics)
- Younger age at cancer therapy
The platinum-induced sensorineural hearing loss develops as an acute toxicity and is generally irreversible and bilateral. Cisplatin and carboplatin are commonly used to treat a variety of childhood cancers (germ cell tumors, brain tumors, hepatoblastoma, soft tissue sarcomas, neuroblastoma). High-frequency hearing is affected first. Cumulative cisplatin doses exceeding 400 mg/m2 and myeloablative doses of carboplatin are associated with severe hearing loss.
Younger age (infants, toddlers) is another factor significantly increasing the risk of ototoxicity. Incidence of severe hearing loss grade 3-4 is significantly higher in children younger than 6 months at the time of cancer treatment.
Radiotherapy to the posterior fossa (inclusive the eighth cranial nerve) is an additional risk factor increases the risk of late ototoxicity in children treated with cisplatin.
Nephrotoxicity
The evidence of long-term renal injury in childhood cancer survivors is limited. Cancer therapy can cause late renal injury several ways:
Surgery: patients who have undergone nephrectomy (mostly due to Wilms tumor or renal carcinoma) are at risk for hyperfiltration injury. Compensatory hypertrophy of the remaining kidney is usually seen. Patients had the higher risk for diminished renal function (reduced glomerular filtration, microalbuminuria, proteinuria), median time from diagnosis to develop renal failure is 10–12 years.
Chemotherapy: the most nephrotoxic agens are cisplatin and carboplatin, ifosfamide, and high-dose methotrexate. Cisplatin can cause tubular damage resulting in diminished GFR and electrolyte wasting (particularly calcium, potassium and magnesium). Carboplatin is less nephrotoxic than cisplatin, important is cumulative dose of carboplatin as a part of pretransplant regimen for solid tumors.
Ifosfamide can cause tubular damage with renal tubular acidosis and Fanconi syndrome (defect of proximal tubulus). Cumulative dose 60–100 g/m2, age less than 5 years and combination with cisplatin/carboplatin increase the risk of ifosfamide associated tubular toxicity. In order to prevent (or reduce) ifosfamide cause nephrotoxicity nephroprotective drugs (uromitexan) and overhydration is recommended.
High dose methotrexate (mainly as a part of therapy for osteosarcoma, non Hodgkinś lymphoma or acute leukemia) can cause acute renal failure dysfunction in approximately 12% of patients. The long-term renal complications have not been reported.
Radiotherapy: to the kidney can result in radiation nephritis after a latent period of 3–12 months. Radiotolerance dose of the kidney is about 20 Gy, so renal parenchyma is relatively radiosensitive. 91% of children who received radiotherapy 24 Gy to abdominal paraaortic lymph nodes develop glomerular dysfunction with decrease creatinine clearence.
The risk and degree of renal dysfunction depend on:
- age of the patient at the time of cancer therapy ( children < 5 years have higher risk)
- type and intensity of therapy
- some factors potentiating nephrotoxicity: sepsis, nephrotoxic antibiotics (vancomycin, amikin…), antimycotic drugs ( amphotericin B), catabolism
- genetic factors (some genetic syndromes lead to chronic renal failure,e.g. Denis-Drash syndrome)
Neurocognitive late effects
Cognitive impairment (learning problems, behavioral changes, loss of concentration, social difficultiesis, long-term education) is one of the most debilitating late effects among childhood cancer survivors, specially in children whose cancer/ its treatment involved the central nervous system.
Incidence of significant neurocognitive problems is variable – not all children with CNS exposure to radiation and chemotherapy will experience neurocognitive effects. There is no certain way to predict which child will experience and severity of neurocognitive problems and which not.
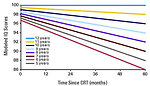
The main risk factor is younger age at the time of cancer treatment and intensity of chemo/radiotherapy (Figure 5).
Neurocognitive late effects are most commonly observed in patients after treatment that require CNS directed therapy. Children in high risk to develop CNS-related cognitive impairments:
- children with primary CNS tumors
- children with leukemia or non-Hodgkin’s lymphoma who receive CNS prophylaxis involving intrathecal chemotherapy and/or radiation therapy
- children with tumors localised in the head and neck (soft tissue sarcomas, neuroblastoma, bone sarcoma) that require radiotherapy
- children treated with whole body radiation and myeloablative chemotherapy as part of a conditioning regimen for allogeneic bone marrow transplantation
Additional risk factors potentiating neurocognitive defect is female gender, younger age at the time of treatment and lower socio-economic status.
Endocrine late effects
Endocrine complications are among the most prevalent late effects experienced by survivors of childhood cancer (affecting 20–50% of survivors).
The most frequent forms ef endocrine late effects are:
- thyroid dysfunction
- obesity
- growth impairment
- precocious/delayed puberty
Thyroid dysfunction is a common late side effect of radiotherapy used for treatment of Hodgkins lymphoma, brain tumors, head and neck tumors (neuroblastoma, parameningeal rhabdomyosarcoma) and cranial irradiation for T-ALL. Thyroid dysfunction may occur in form of hypothyroidism, hyperthyroidism, goiter or nodular changes in thyroid parenchyma. Therefore monitoring of thyroid hormons and ultrasound of thyroid is a standard part of follow up visit at least once a year for survivors with risk of thyroid dysfunction.
Growth hormone deficiency is caused by cranial irradiation especially due to brain tumors, parameningeal soft tissue sarcomas. Usually is not isolated, but occur together with hypopituitarism. Severity of hormonal deficiency depends on dose and volume of radiation, age at time of radiation exposure and gender. Linear growth (height) may be inhibited by cranial irradiation through its effect on the hypothalamic/pituitary axis. Weight and height assessment and comparing with standard scale is essentials part of follow up visit.
Obesity is one of the big challenges for childhood cancer survivors due to further associated problems – low physical activity, metabolic syndrome, risk of diabetes, hypertension and secondary hormonal changes. Therefore is important to pay attention to the obese adolescents and young adults and to assess weight as a standard part of periodic follow up visit. Interventions to prevent obesity and promote physical activity have been shown to reduce cardiovascular morbidity and mortality and improve quality of life (Figure 6).
Osteoporosis (decreased bone mineral density) is a challenge in childhood cancer survivors, especially for patients with brain tumors, acute leukemia, lymphoma, soft tissue sarcomas or Ewing sarcomas. Risk factors include gonadal dysfunction, male gender and prior cranial irradiation. Treatment with corticosteroids, methotrexate and long-term immobilisation may impair mineralization. Patients who experienced osteoporosis are at increased risk for pathologic fractures. Long-term survivors should be periodically assessed to determine risk for osteoporosis (densitometry at least every 2–3 years).
Fertility
The risk of infertility is generally related to the type and localisation of primary tumor and type, dose and combination of therapy. Treatment with radiation therapy and/or chemotherapy may have adverse effects on:
- germ cells (i.e. ova and sperm)
- stromal gonadal cells and gonadal endocrine functions
Surgery (orchiectomy or oophorectomy) for gonadal germ cell tumors (testis, ovary) itself usually do not affect fertility significantly. In combination with chemotherapy the risk of infertility is much higher.
Chemotherapy (specially alkylating agents) may be associated with high risk of infertility. Factors influencing risk of gonadal injury:
- cumulative dose and type of chemotherapy
- prepubertal and pubertal age at the time of treatment
- gender
- lenght of chemotherapy
Radiotherapy: mechanism of action and its effect on fertility and gonadal function impairment is dose, age- and gender-specific.
Radiation dose of 10–20 Gy in boys leads to permanent azoospermia. Lower dose (<5 Gy) can cause reversible spermatogenesis failure (duration is usually 2–3 years). Radiation dose over 20 Gy in prepubertal boys and 25–30 Gy in postpubertal boys is toxic for Leydig cells (they are less radiosensitive than germ cells) with subsequent inadequate production of testosteron and delayed sexual maturation.
Ovarian tissue in girls is very radiosensitive.Radiation to the ovaries leads to both – sterilization and hormonal deficiency. Radiation dose > 2Gy reduce the follicle pool about 50%. Changes caused by radiation dose 4–7 Gy to ovaries are irreversible. Radiation to the pelvis 14–30 Gy cause decresed vascularisation and elasticity of the uterus with increased risk of miscarriages, pre-term birth, fetal malposition and low-birth weight of offsprings.
Males: treatment with alkylating drugs chemotherapy (cyclophosphamide, dacarbasine, ifosfamide, busulphan melphalan, temozolomide) for at least 3 month duration may cause oligo or azoospermia. Recovery of spermatogenesis is posible in about 20 %. Hormonal production of androgens by Leydig cells usually maintain normal. Pubertal and postpubertal boys are offered the option of sperm cryopreservation prior to treatment after inform concern is subscribed. For immature prepubertal boys with intensive therapy and high risk of infertility is the only chance biopsy of the testis with testicular tissue cryopreservation.
Females: situation regarding fertility impairment is more complicated in adolescent girls and young females than in boys. Infertility is usually associated with significant psychosocial deprivation. Chemotherapy (mainly alkylating agens) leads to:
- toxicity of primordial follicle reserve with consequent premature ovarian failure and premature menopause (Figure 7)
- toxicity to growing follicle leads to temporary amenorrhea
Discussion regarding fertility preservation and family planning is important because the adolescent girls and young females may be fertile for only a relatively short period of time. Fertility preservation in pediatric and pubertal age girls is complicated from several reasons:
- toxicity to growing follicle leads to temporary amenorrhea
- biology behavior of pediatric tumors do not allowed to postpone effective treatment (therefore hormonal stimulation and cryoconservation of oocytes is usually not possible to perform)
- pubertal and postpubertal patients usually do not have sexual partner for planning family (therefore embryo cryoconservation is not performed)
- biopsy and cryoconservation of ovarian tissue is possible method of fertility preservation for prepubertal and pospubertal girls
- in case of radiotherapy to the pelvis is indicated, transposition of one ovary and cryopreservation of ovarian tissue of contralateral ovary is treatment of choice for fertility preservation
- pharmacology ovarian protection by application of gonadoliberin analogues (dipherellin) is still experimental (Figure 8)
Musculoskeletal late effects, growth failure
Patients’ height is affected by premorbid growth, familial and genetic predispositions. Growth failure shoud be permanent or progresive in time, proportional or disproportional.
Mechanisms of growth failure are:
- growth hormone deficiency
- peripheral tissue resistance to growth hormone
- combined mechanisms
- unknown
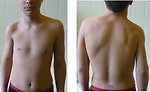
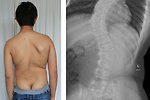
Radiotherapy to the brain and spine may inhibit linear growth (height) through its effect on the hypothalamic/pituitary axis. The effect of radiotherapy is dose, volume and age dependent. Patients with brain tumors treated with large doses of whole-brain or craniospinal radiotherapy are the most affected group. Lower doses of radiation (e.g. CNS prophylaxis for T-cell leukemia) may result in less dramatic retardation of growth. Children treated with total body irradiation as a part of conditioning regimen and high-dose chemotherapy (high risk neuroblastoma) usually have severe growth retardation. Radiotherapy also can cause soft tissue hypoplasia (Figure 9) or avascular necrosis (especially hip joint). Asymmetric radiation exposure can result in differential growth and lead to functional disabilities, pain, asymmetric appearance and scoliosis. (Figure 10) These effects of radiation may not be apparent at the end of therapy but with growth, especially during the pubertal growth spurt, become more evident.
Chemotherapy mainly high dose chemotherapy directly affects bone growth. Younger age, type and cumulative dose of chemotherapy are the main risk factors. Treatment regimens for ALL, lymphomas, brain tumors, Wilms’ tumor, and sarcomas make survivors particularly vulnerable to musculoskeletal effects.
Secondary malignancies
The cumulative risk of subsequent malignant cancer is 5 to 20 times greater than that expected in the general population, with median time interval 10–20 years since the first cancer treatment.
Factors influencing risk for secondary malignant tumor:
- type of primary tumor
- age of the patient at the time of diagnosis of primary cancer
- type and intensity of previous cancer treatment
- combined chemo and radiotherapy
- genetic factors
- environmental exposure (professional exposure)
- life style factors (sun light, stress, chemicals, smoking, diet etc)
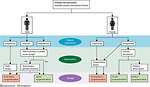
Travis et al. in 2012 defined 3 groups of secondary cancer according to predominant risk factors:
- treatment-related
- genetic syndromes related
- mixed (Figure 11)
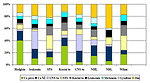
According to results of CCSK (Children Cancer Survival Study) in USA the secondary malignancies are the leading cause of non-relapse late mortality and represent 16 % of all malignancies diagnosed in entire population (Figure 12). The main goal of secondary malignancies study is to identify the group of the patients with the highest risk to develop secondary cancer.
Treatment related factors: radiation therapy for pediatric tumors is associated with the development of subsequent cancer and depend on total dose of radiation, irradiated field and period of follow up. The typical example is Hodgkin lymphoma with involved field irradiation. Patients with Hodgkin lymphoma are at risk to develop secondary thyroid cancer, malignant melanoma of the skin and girls are at risk to develop breast cancer.
Certain types of chemotherapy (alkylating agents, topoisomerase II inhibitors) are associated with risk to develop secondary hematologic malignancies (leukemia). One of the most important factors is total cumulative dose of chemotherapy agents.
Certain secondary malignancies are asscociated with certain primary tumors and its treatment. These informations are important to know for health care providers to plan long-term follow up and care of childhood cancer survivors (Figure 13).
Genetic predisposition: some young adult childhood cancer survivors are at increased risk of secondary malignancy because they have a genetic form of a disease that predisposes them to other cancers (typical example is hereditary retinoblastoma with germinal mutation of RB1 gene and risk as high as 50 % of subsequent secondary osteosarcoma within 10–15 years). Other rare syndromes predispose survivors to second malignancies are Li-Fraumeni syndrome (TP53 mutation, different types of tumors), familial adenomatous polyposis (germinal mutation of FAB gene and primary hepatoblastoma and very high risk of subsequent colon adenocarcinoma).
Environmental factors and life style: Skin is sensitive to radiation carcinogenesis, especially at a young age. Doses of radiation used in the treatment of childhood cancers, such as Hodgkin’s and non-Hodgkin’s lymphomas, soft tissue sarcoma, and Wilms’ tumor, are associated with an increased risk for basal cell carcinoma and squamous cell carcinoma rule for early detection of skin cancer, and the importance of periodic examination of the skin in and around the radiation field. Among the general population, public education regarding sun protection and self-examination has been associated with earlier stage of disease at diagnosis.
Psychological and behavioral late effects
Childhood cancer survivors are at risk of developing symptoms of psychologic distress. Cancer and its treatment may have psychological, behavioral and social effects (so called “sword of Damokles”). About 25 % of childhood cancer survivors experience some form of depression or anxiety, significant fear of relapse of the tumor or new secondary cancer.
A syndrome similar to post-traumatic stress disorder (PTSD) has been reported in 5 to 20% of childhood cancer survivors. There are some survivors who remain seriously troubled and are impaired by their psychological problems.They are worried about many aspects of survivorship (the risk of relapse, dying, another chemotherapy, potential problems with sexuality and fertility, partnership, self body image, school and work performance, social relationship etc.). Fortunately, most childhood cancer survivors achieve normal psychological and social status.
Author: Viera Bajčiová, MD, PhD