Emergencies in pediatric oncology Tumor lysis syndrome
Definition
Tumor lysis syndrome (TLS) is an oncologic emergency that is caused by massive tumor cell lysis with the release of large amounts of potassium, phosphate, and nucleic acids into the systemic circulation. Catabolism of the nucleic acids to uric acid leads to hyperuricemia, and the marked increase in uric acid excretion can result in the precipitation of uric acid in the renal tubules and can also induce renal vasoconstriction, impaired autoregulation, decreased renal blood flow, and inflammation, resulting in acute kidney injury. Hyperphosphatemia with calcium phosphate deposition in the renal tubules can also cause acute kidney injury.
- Laboratory TLS is defined as any two or more of the following metabolic abnormalities and presents within three days before or seven days after instituting chemotherapy: hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.
- Clinical TLS is defined as laboratory TLS plus one or more of the following that was not directly or probably attributable to a therapeutic agent: increased serum creatinine concentration (≥1.5 times the ULN), cardiac arrhythmia/sudden death, or a seizure.
Pathogenesis
TLS most often occurs after the initiation of cytotoxic therapy in patients with high-grade lymphomas (particularly the Burkitt subtype) and acute lymphoblastic leukemia. However, TLS can occur spontaneously and with other tumor types that have a high proliferative rate, large tumor burden, or high sensitivity to cytotoxic therapy.
This releases massive quantities of intracellular contents (potassium, phosphate, and nucleic acids that can be metabolized to uric acid) into the systemic circulation. The metabolic consequences include hyperkalemia, hyperphosphatemia, secondary hypocalcemia, hyperuricemia, and acute kidney injury. High levels of both uric acid and phosphate increase the severity of acute kidney injury because uric acid precipitates readily in the presence of calcium phosphate crystals, and calcium phosphate precipitates readily in the presence of uric acid crystals.
Hyperuricemia
Hyperuricemia is a consequence of the catabolism of purine nucleic acids to hypoxanthine and xanthine and then to uric acid via the enzyme xanthine oxidase (Figure 1). Uric acid is poorly soluble in water, particularly in the usually acidic environment in the distal tubules and collecting system of the kidney. Overproduction and overexcretion of uric acid in TLS can lead to crystal precipitation and deposition in the renal tubules, and acute uric acid nephropathy with acute kidney injury.
Hyperphosphatemia
The phosphorus concentration in malignant cells is up to four times higher than in normal cells. Thus, rapid tumor breakdown often leads to hyperphosphatemia which can cause secondary hypocalcemia. When the calcium concentration times phosphate concentration (the calcium phosphate product) exceeds 60 mg2/dL2, there is an increased risk of calcium phosphate precipitation in the renal tubules, which can lead to acute kidney injury. In addition, precipitation in the heart may lead to cardiac arrhythmias.
Clinical manifestation
The symptoms associated with TLS largely reflect the associated metabolic abnormalities (hyperkalemia, hyperphosphatemia, and hypocalcemia). They include nausea, vomiting, diarrhea, anorexia, lethargy, hematuria, heart failure, cardiac dysrhythmias, seizures, muscle cramps, tetany, syncope, and possible sudden death.
Acute uric acid or calcium phosphate deposition does not usually cause symptoms referable to the urinary tract, although flank pain can occur if there is renal pelvic or ureteral stone formation. The urinalysis classically shows many uric acid crystals or amorphous urates in an acid urine (Figure 2), but is occasionally relatively normal due to lack of output from the obstructed nephrons.
Risk factors
The risk of TLS is greatest in patients treated for hematologic malignancies but is not uniform among these disorders. Certain intrinsic tumor-related factors are associated with a higher risk. These include:
- High tumor cell proliferation rate
- Chemosensitivity of the malignancy
- Large tumor burden, as manifested by bulky disease >10 cm in diameter and/or a white blood cell count >50,000 per microL, a pretreatment serum lactate dehydrogenase (LDH) more than two times the upper limit of normal, organ infiltration, or bone marrow involvement
There are also clinical features that predispose to the development of TLS:
- Pretreatment hyperuricemia (serum uric acid >7.5 mg/dL [446 micromol/L]) or hyperphosphatemia
- A preexisting nephropathy or exposure to nephrotoxins
- Oliguria and/or acidic urine
- Dehydration, volume depletion, or inadequate hydration during treatment
Risk stratification
HIGH RISK (>5 percent risk of TLS)
- stage III or IV Burkitt, ALL with a WBC ≥100,000/uL, AML with WBC ≥100,000/uL, stage III or IV lymphoblastic lymphoma
- Patients with intermediate risk disease with renal dysfunction and/or renal involvement, or uric acid, potassium, or phosphate levels above the ULN
INTERMEDIATE RISK (risk of TLS 1 to 5 percent)
- Stage III or IV childhood anaplastic large cell lymphoma, early stage Burkitt lymphoma, ALL with WBC <100,000/uL, AML with WBC 25,000 to 100,000/uL, chronic lymphocytic leukemia
- Rare bulky solid tumors that are highly sensitive to chemotherapy (such as neuroblastoma, germ cell cancer, small cell lung cancer)
LOW RISK (risk for TLS <1)
- AML with WBC <25,000/microL, Multiple myeloma and CML
- Other solid tumors
TLS prevention
IV hydration
Aggressive intravenous (IV) hydration is the cornerstone of preventing tumor lysis syndrome (TLS) and is recommended prior to therapy in all patients at intermediate or high risk for TLS. The goal of IV hydration is to improve renal perfusion and glomerular filtration, and induce a high urine output to minimize the likelihood of uric acid or calcium phosphate precipitation in the tubules.
Both children and adults at risk for TLS initially receive 2 to 3 L/m2 per day of IV fluid (or 200 mL/kg per day in children weighing ≤10 kg)
Recomended hydration fluid
- 5 percent dextrose one-quarter normal (isotonic) saline
- In patients with hyponatremia or volume depletion, isotonic saline should be the initial hydration fluid
- Due to the risk of hyperkalemia and hyperphosphatemia with calcium phosphate precipitation once tumor breakdown begins, potassium and calcium should be withheld from the hydration fluids, at least initially
Urine output should be monitored closely and maintained within a range of 80 to 100 mL/m2 per hour (2 mL/kg per hour for both children and adults, 4 to 6 mL/kg per hour if ≤10 kg)
Diuretics can be used to maintain the urine output, if necessary, but should not be required in patients with relatively normal renal and cardiac function. Use of diuretics is contraindicated in patients with hypovolemia or obstructive uropathy.
- Loop diuretics such as furosemide appear preferable because they not only induce diuresis, but may also increase potassium secretion.
Hypouricemic agents
Allopurinol
Allopurinol is a hypoxanthine analog that competitively inhibits xanthine oxidase, blocking the metabolism of hypoxanthine and xanthine to uric acid (Figure 1). Allopurinol effectively decreases the formation of new uric acid and reduces the incidence of obstructive uropathy in patients with malignant disease at risk for TLS. It is inexpensive and orally administered, and thus preferred for patients with a low risk of TLS.
Dose and administration — The usual allopurinol dose in adults is 100 mg/m2 every eight hours (maximum 800 mg per day). In children, the dose is 50 to 100 mg/m2 every eight hours (maximum 300 mg/m2 per day) or 10 mg/kg per day in divided doses every eight hours. The dose must be reduced by 50 percent in the setting of acute kidney injury due to potential for accumulation of allopurinol and metabolites.
Rasburicase
An alternative approach to allopurinol for lowering serum uric acid levels is to promote the degradation of uric acid by the administration of urate oxidase (uricase), which catalyzes oxidation of uric acid to the much more water-soluble compound allantoin (Figure 1). Urate oxidase is present in most mammals but not humans.
Rasburicase is well tolerated, rapidly breaks down serum uric acid, and is effective in preventing and treating hyperuricemia and TLS. This rapid reduction in serum uric acid is in contrast to the effect of allopurinol, which decreases uric acid formation and therefore does not acutely reduce the serum uric acid concentration.
Dosing and administration
- High-risk patients or a baseline uric acid level >7.5 mg/dL (446 micromol/L) – rasburicase 0.2 mg/kg
- Intermediate-risk patients with baseline uric acid ≤7.5 mg/dL – rasburicase 0.15 mg/kg
Hemolysis in patients with G6PD deficiency – Rasburicase is CONTRAINDICATED in patients with G6PD deficiency because hydrogen peroxide, a byproduct of uric acid breakdown, can cause severe hemolysis in this setting. Patients being considered for rasburicase (especially males) who have the potential for G6PD deficiency by virtue of a history of prior drug-induced hemolytic anemia and/or a racial/ethnic background associated with G6PD deficiency (eg, African-American, Mediterranean, or Southeast Asian descent) should undergo definitive quantitative enzyme assay or genetic testing followed by quantitative enzyme assay if appropriate.
Treatment of establised TLS
Electrolyte abnormalities
Hyperkalemia
- Hyperkalemia is the most dangerous component of TLS because it can cause sudden death due to cardiac dysrhythmias. Patients should limit potassium and phosphate intake during the risk period for TLS
- frequent measurement of serum potassium (every four to six hours)
- continuous cardiac monitoring
- administration of oral sodium polystyrene sulfonate are recommended in patients with TLS and acute kidney injury
- Glucose plus insulin or beta-agonists can be used as temporizing measures
- calcium gluconate may be used to reduce the risk of cardiac dysrhythmia
- If needed, hemodialysis and hemofiltration effectively removes potassium.
Symptomatic hypocalcemia
- Symptomatic hypocalcemia should be treated with calcium at the lowest doses required to relieve symptoms
- To avoid calcium-phosphate precipitation, most symptomatic acutely hypocalcemic patients with hyperphosphatemia due to TLS (particularly if the calcium phosphate product is >60 mg2 per dL2) should not be treated with calcium until hyperphosphatemia is corrected.
- Asymptomatic patients with hypocalcemia do not require treatment.
Hyperphoshatemia
Hyperphosphatemia remains a major problem in TLS and can cause acute kidney injury. Strategies aimed at lowering serum phosphate levels (aggressive hydration and phosphate binder therapy) should be used in conjunction with control of uric acid in patients who have established TLS or who are at high risk of developing TLS.
Indications for renal replacement therapy
Despite optimal care, severe acute kidney injury develops in some patients, requiring renal replacement therapy. The need for dialysis during induction therapy for high-risk hematologic malignancies has substantially declined since the introduction of rasburicase.
Indications for renal replacement therapy are similar to those in patients with other causes of acute kidney injury, although somewhat lower thresholds are used for patients with TLS because of potentially rapid potassium release and accumulation, particularly if urine output is low.
- Among the indications for renal replacement therapy in patients with TLS are:
- Severe oliguria or anuria
- Persistent hyperkalemia
- Hyperphosphatemia-induced symptomatic hypocalcemia
A calcium-phosphate product ≥70 mg2/dL2
Author: Pavel Mazánek, MD
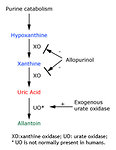 Figure 1: Endogenous production of uric acid |
 Figure 2: Urine of a patient with ALL and TLS |
||||||
